We use a reductionist approach to study the neurobiology of appetite. We focus on the mechanisms of hunger and compare these across other behavioral states, such as thirst, fear, and even addiction. We developed CaRMA (Calcium and RNA Multiplexed Activity) imaging to accelerate understanding of how the cell types in the brain encode and influence different behavioral states. To bridge the gap between research in model organisms and human disease, we use chemistry and protein engineering to create new chemogenetic technologies.
Appetite

Our research investigates how the brain controls appetite. To identify the neural circuits that motivate goal-directed behaviors related to hunger, thirst, and stress, we study areas throughout the brain. Critical to this effort, the lab develops cutting-edge deep-brain calcium imaging methods to build a systematic framework for the different phases of motivated behaviors. Our goal is to use detailed molecular and cellular models of appetite to develop mechanism-based treatment strategies for obesity and other neurological disorders.
Using these approaches we have discovered different neurons responsible for the unpleasant feelings of hunger and thirst as well as different neurons for the pleasantness of palatable food and beverages. We are developing models for hunger-motivated behaviors based on in vivo neural activity recordings to identify neural algorithms controlling each phase of appetite.
We have also uncovered molecular, cellular, and synaptic specializations of neurons involved in long-timescale functions, such as energy homeostasis. This work shows that neurons controlling biological processes over hours to days have unique characteristics relative to conventional neurons that influence fast-timescale biological processes.
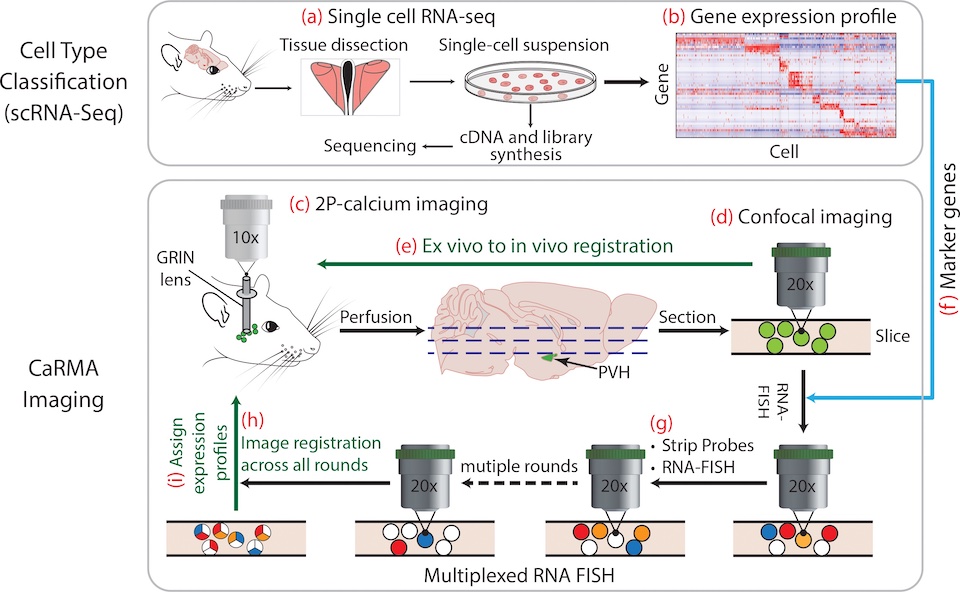
CaRMA Imaging
To seamlessly integrate information from molecular and systems neuroscience, we developed the CaRMA (Calcium and RNA Multiplexed Activity) imaging approach. This involves in vivo imaging of calcium dynamics without regard to cell type followed by ex vivo analysis of gene expression in the same neurons using multiplexed RNA-FISH. We are using CaRMA imaging to deconstruct the role of neuron types in different behavioral states. To streamline the CaRMA imaging experimental pipeline, we have developed EASI-FISH in collaboration with Dr. Paul Tillberg as Janelia. EASI-FISH allows multi-round RNA-FISH in thick tissue sections, which means that brain tissue does not need to be subsectioned for FISH after in vivo calcium imaging.
Chemogenetics

Chemogenetics is an approach to achieve cell type-specific pharmacological perturbations in the nervous system of behaving animals by installing an engineered receptor that is activated by a highly selective small molecule drug. Traditionally, experimenters and clinicians study brain function and treat disease states with drugs that target receptors on cells. However, many cells usually express a given receptor, which reduces specificity in research applications and causes side effects when a drug is used therapeutically.
By targeting chemogenetic receptors to specific neuron populations, its cognate ligand can control the activity of only that population, turning it ‘on’ or ‘off’. This allows experimenters to assess the role of a single neuron population in a certain behavior or disease state without off-target effects from other neuron populations. Chemogenetics has not yet been applied as a therapy for human diseases, so we have developed a chemogenetic receptor for the FDA-approved drug,, varenicline, which is currently under investigation as a potential drug-gated gene therapy. We have also developed novel ligands(available here and here) for these chemogenetic receptors for research purposes.